Question 1.
What is a balanced chemical equation? Why should chemical equations be balanced?
Answer:
1) A chemical equation in which the number of atoms of different elements on the reactants side (left side) are same as those on product side (right side) is called a balanced chemical equation.
Ex : Zn + 2HCl → ZnCl2+ H2↑
2) All the chemical equations must balance because atoms are neither created nor destroyed in chemical reactions.
3) The number of atoms of each element before and after reaction must be the same.
4) According to the law of conservation of mass, the total mass of the substances that are taking part in a chemical reaction must be the same before and after the reaction.
Question 2.
Balance the following chemical equations.
a) NaOH + H2SO4→ Na2SO4+ H2O
b) Hg (NO3)2+ KI → Hgl2+ KNO3
c) H2+ O2→ H2O
d) KClO3→ KCl + O2
e) C3H8+ O2→ CO2+ H2O
Answer:
a) 2NaOH + H2SO4→ Na2SO4+ 2H2O
b) Hg (NO3)2+ 2 KI → Hgl2+ 2KNO3
c) 2H2+ O2→ 2H2O
d) 2KClO3→ 2KCl + 3O2
e) C3Hg + 5O2→ 3CO2+ 4H2O
Question 3.
Write the balanced chemical equations for the following reactions.
a) Zinc + Silver nitrate → Zinc nitrate + Silver
b) Aluminium + Copper chloride → Aluminium chloride + Copper
c) Hydrogen + Chlorine → Hydrogen chloride
d) Ammonium nitrate → Nitrous Oxide + Water
Answer:
a) Zn + 2AgNO3→ Zn(NO3)2+ 2Ag
b) 2Al + 3CuCl2→ 2AlCl3+ 3Cu
c) H2+ Cl2→ 2HCl
d) NH4NO3→ N2O + 2H2O
Question 4.
Write the balanced chemical equations for the following and identify the type of reaction in each case.
a) Calcium hydroxide(aq) + Nitric acid(aq) → Water(l) + Calcium nitrate(aq)
b) Magnesium(sJ + Iodine → Magnesium Iodide(s)
c) Magnesium(s) + Hydrochloric acid(aq) → Magnesium chloride(aq) + Hydrogen^
d) Zinc(s) + Calcium chloride(aq) → Zinc Chloride(aq) + Calcium(s)
Answer:
a) Ca(OH2) + HNO3→ H2O + Ca(NO3)2
This is double decomposition reaction.
b) Mg + I2→ Mgl2
This is chemical combination reaction.
c) Mg + 2HCl → MgCl2+ H2↑
This is chemical displacement reaction.
d) Zn + CaCl2→ ZnCl2+ Ca
This is chemical displacement reaction. This reaction is not possible, because calcium is more reactive than zinc.
Question 5.
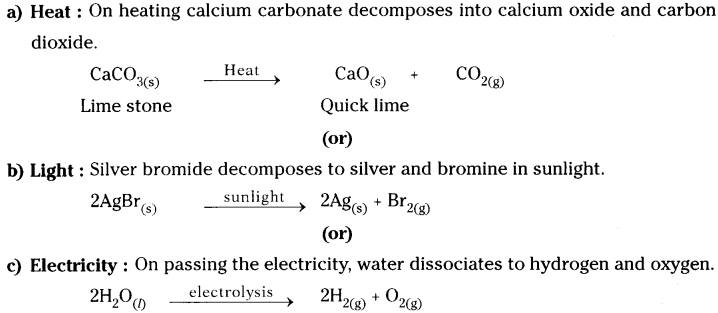
Write an equation for decomposition reaction where energy is supplied in the form of heat / light / electricity.
Answer:
Chemical decomposition reaction : A chemical reaction in which a single substance splits into two or more substances is called chemical decomposition.
For decomposition reaction energy is supplied in the form of :
Question 6.
What do you mean by precipitation reaction?
Answer:
A reaction in which insoluble substance in water is formed as product is called precipitation reaction.
Question 7.
How does chemical displacement reactions differ from chemical decomposition reaction? Explain with an example for each.
Answer:
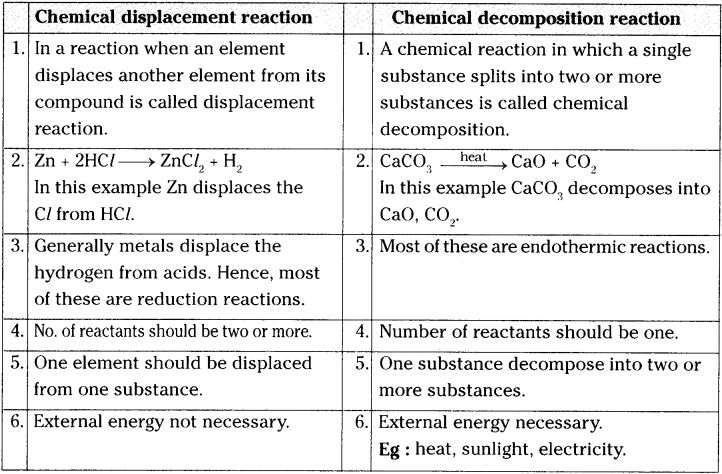
Question 8.
Name the reactions taking place in the presence of sunlight.
Answer:
1) In the presence of sunlight plants prepare their food by taking C02 from the air and H20 from the soil with their chloroplasts of the green leaves. This reaction is called photosynthesis.
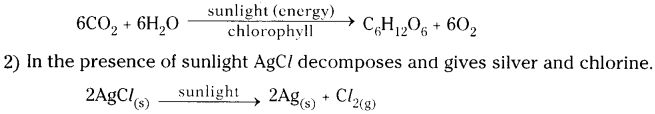
Question 9.
Why does respiration considered as an exothermic reaction? Explain.
Answer:
- We need energy to stay alive.
- We get this energy from food we eat.
- During digestion, food is broken down into simpler substances.
- For example, rice and potato contains starch.
- The starch breaks down to form glucose.
- This glucose combines with oxygen in the cells of our body and releases energy, which helps to do the various works.
- During this process, energy is given out. Hence this reaction can be called exothermic reaction.
- The special name of this reaction is respiration.
- So respiration is considered as exothermic reaction.
- C6H12O6+ 6O2→ 6CO2+ 6H2O + Q (Energy)
Question 10.
What is the difference between displacement and double displacement reactions? Write equations for these reactions.
Answer:
| Chemical displacement reaction |
Chemical double displacement reaction |
| 1. In a reaction when an active element displaces less active element from its compound is called displacement reaction. |
1. If two reactants exchange their constituents chemically and form two products, then the reaction is called as double displacement reaction. |
2. Zn + 2HCl → ZnCl2+ H2
In this example Zn displaces Hydrogen from HCl. |
2. Na2SO4+ BaCl2→ BaSO4+ 2NaCl
In this reaction SO42-and Cl-are mutually exchanged. |
3. General formula to the reaction is
A + BC → AC + B |
3. General formula to the reaction is
AB + CD → AD + BC |
Question 11.
MnOz + 4HCl → MnCl2+ 2H2O + Cl2↑
In the above equation, name the compound which is oxidized and which is reduced.
Answer:
In the above equation, HCl compound is oxidized and MnO2is reduced.
Question 12.
Give two examples for oxidation-reduction reaction.
Oxidation :
Oxidation is a reaction that involves the addition of oxygen or loss of hydrogen or electrons.
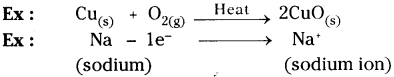
Reduction :
The process in which a substance loses oxygen or gains hydrogen or electrons is known as reduction.
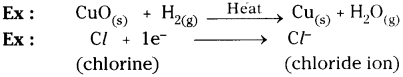
Question 13.
In the refining of silver, the recovery of silver from silver nitrate solution involved displacement by copper metal. Write the reaction involved.
Answer:
- Cu(s)+ 2 AgNO3(aq)> Cu(NO3)2(aq)+ 2Ag(s)
- This is redox reaction, copper is the reducing agent and the silver is reduced.
- Electrons from the copper metal are transferred to the silver.
- This reaction can also be called a displacement reaction because copper displaces silver as it is more reactive.
Question 14.
What do you mean by corrosion? How can you prevent it?
Answer:
- When some metals are exposed to moisture, acids, etc. they tarnish due to the formation of respective metal oxide on their surface. This process is called “corrosion”.
- Corrosion can be prevented by painting, oiling, greasing, galvanizing, chrome plating or making alloys.
- Galvanizing is a method of protecting iron from rusting by coating them a thin layer of zinc.
Question 15.
Explain rancidity.
Answer:
- Oxidation reactions in food material that were left for a long period are responsible for spoiling of food. This process is called “rancidity”.
- When these processes occur in food, undesirable odours and flavours can result.
- Rancidity is an oxidation reaction.
Question 16.
Balance the following chemical equations including the physical states,
a) C6H12O6→ C2H5OH + CO2
b) Fe + O2→ Fe2O3
c) NH3+ Cl2→ N2H4+ NH4Cl
d) Na + H2O → NaOH + H2
Answer:
a) C6H12O6(s)→ C2H5OH(l)+ CO2(g)
b) Fe(s)+ O2(g)→ Fe2O3(s)
c) NH3(aq)+ Cl2(g)→ N2H4(l)+ NH4Cl(aq)
d) 2Na(s)+ 2H2O(l)→ NaOH + H2
Question 17.
Balance the chemical equation by including the physical states of the substances for the following reactions.
a) Barium chloride and Sodium sulphate aqueous solutions react to give insoluble Barium sulphate and aqueous solution of Sodium chloride.
b) Sodium hydroxide reacts with Hydrochloric acid to produce Sodium chloride and water.
c) Zinc pieces react with dilute Hydrochloric acid to liberate Hydrogen gas and forms Zinc chloride.
Answer:
a) BaCl2(aq)+ Na2SO4(aq)→ BaSO4(s)↓ + NaCl(aq)
b) NaOH(aq)+ HCl(aq)→ NaCl(aq)+ H2O(l)
c) Zn(s)+ 2HCl(aq)→ ZnCl2(aq)+ H2(g)
Question 18.
A shiny brown coloured element ‘X’ on heating in air becomes black in colour. Can you predict the element ‘X’ and the black coloured substance formed? How do you support your predictions?
Answer:
The brown coloured element is copper (Cu). On heating copper reacts with oxygen
present in the atmosphere to form copper oxide which is black in colour.
The reaction is shown below.
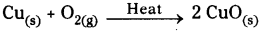
If we pass hydrogen gas over hot copper oxide we will notice that black coating on copper turns brown because copper oxide loses oxygen to form copper.
This will support our prediction.
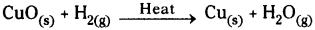
Question 19.
Why do we apply paint on iron articles?
Answer:
- Ferrous reacts with oxygen in the air and form iron oxide.
2 Fe + O2→2 FeO
- This reaction is called corrosion. It spoils the iron articles by rusting.
- Corrosion of iron articles can be prevented or minimized by shielding the metal surface from oxygen and moisture.
- It can be prevented by applying paint on the articles.
Question 20.
What is the use of keeping food in air tight containers?
Answer:
- Oxidation is defined as the interaction of oxygen molecules with all the different substances from metal to living tissue which may come into contact with it.
- When fats and oils are oxidized they become rancid. Their smell and taste change.
- Keeping food in airtight containers helps to slow down oxidation process.
- So, manufacturers of potato chips usually flush bags of chips with gas such as nitrogen to prevent the chips from getting oxidized.